Abstract
Background
Prognosis of patients with relapsed or refractory AML has been poor as no standard salvage therapy exists. Most trials of investigational agents begin in r/r AML and accrue a wide range of patients with different characteristics. Historical context for outcomes can be used as a reference for the development of future protocols and novel agents. The objective of this study was to evaluate outcomes of patients presenting with r/r AML in a single institution.
Methods
We analyzed the outcomes of patients who were treated for r/r AML for at least one treatment course at our institution between the years 2002 and 2016. At the time of inclusion in this study, patients had at least one prior treatment failure, were ≥18 years old at time of AML diagnosis, and had no central nervous system (CNS) involvement. Patients with acute promyelocytic leukemia were excluded.
Descriptive characteristics of patients were summarized by proportions. Complete remission (CR) and complete remission with incomplete hematologic recovery (CRi) rates were described as proportion with Wald 95% confidence intervals. Time to event analyses was estimated using the Kaplan-Meier method. Univariate and multivariate Cox models estimated p-values from Wald chi-square.
Results
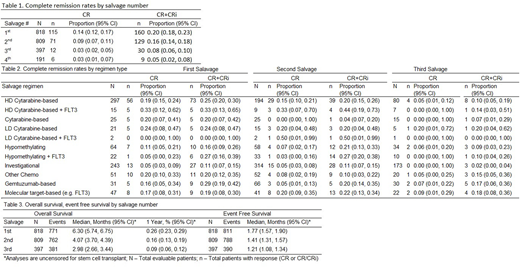
A total of 1021 patients were included. Median age was 60 years (range, 18 - 87). At least one cytogenetic abnormality was present in 53.3% (n=546) of the population, 34.5% (n=352) had a history of an antecedent hematologic disease, and 10.5% (n=107) were therapy-related AML. For patients with available induction records, approximately 46% (295/635) were refractory. Among patients who achieved a CR after induction, 45% (118/264) had a CR duration <6 months.
Overall, only a small proportion of all r/r AML patients achieved a CR2 and CR rates decreased with each subsequent salvage attempt (Table 1). Rates were lower among patients >60 years of age (1st salvage p=0.05, 2nd salvage p=0.003, 3rd salvage p=0.09). Among various types of salvage regimens, the range of CR was from 0 to 36%. Regimens based on high dose cytarabine (HDAC) were the most common (n=297). Although sample size was modest, HDAC regimens that additionally contained a FLT3 inhibitor induced the highest CR and CR/CRi rates (33% CR2, 44% CR3) (Table 2). Age (p=0.0006), cytogenetics (p<0.001), antecedent hematologic disease (p<0.001), duration of first remission (p<0.001), and year of relapse (p<0.001) were associated with CR in univariate analyses. In multivariate analyses, cytogenetics (p=0.004), antecedent hematologic disease (p<0.001), year of relapse (p=0.002), and platelet count (p=0.003) were associated with CR.
Overall survival and event free survival were modest and decreased with subsequent salvage (Table 3). Age (p<0.001), cytogenetics (p<0.001), antecedent hematologic disease (p<0.001), de novo/therapy-induced AML (p=0.007), duration of first remission (p<0.001), and platelet count (p<0.001) were associated with survival in univariate analyses. In multivariate analyses, age (p<0.001), cytogenetics (p<0.001), antecedent hematologic disease (p<0.001), duration of first remission (p=0.002), platelet count (p=0.005) and blast count (p=0.014) were associated with survival.
Conclusion
Overall, most patients were unable to achieve a CR2+. Fewer patients were able to achieve subsequent CR after a second treatment failure or relapse. EFS was short due to most patients failing to achieve CR. Even at first salvage, OS was less than 1 year. These data demonstrate that patients with relapsed or refractory AML have poor overall outcomes and further emphasizes the need for additional options for these patients. These data can be used to help guide the development of novel therapeutic options in AML.
Ravandi:Sunesis: Honoraria; Jazz: Honoraria; Macrogenix: Honoraria, Research Funding; Abbvie: Research Funding; Jazz: Honoraria; Orsenix: Honoraria; Bristol-Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Xencor: Research Funding; Bristol-Myers Squibb: Research Funding; Orsenix: Honoraria; Astellas Pharmaceuticals: Consultancy, Honoraria; Abbvie: Research Funding; Sunesis: Honoraria; Seattle Genetics: Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Xencor: Research Funding; Amgen: Honoraria, Research Funding, Speakers Bureau; Amgen: Honoraria, Research Funding, Speakers Bureau; Macrogenix: Honoraria, Research Funding. Kadia:Pfizer: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; BMS: Research Funding; Abbvie: Consultancy; Takeda: Consultancy; Novartis: Consultancy; Jazz: Consultancy, Research Funding; BMS: Research Funding; Celgene: Research Funding; Takeda: Consultancy; Novartis: Consultancy; Amgen: Consultancy, Research Funding; Jazz: Consultancy, Research Funding; Celgene: Research Funding; Abbvie: Consultancy. DiNardo:Celgene: Honoraria; Abbvie: Honoraria; Bayer: Honoraria; Agios: Consultancy; Medimmune: Honoraria; Karyopharm: Honoraria. Daver:Pfizer: Consultancy; Novartis: Research Funding; Daiichi-Sankyo: Research Funding; Karyopharm: Consultancy; ARIAD: Research Funding; Pfizer: Research Funding; Kiromic: Research Funding; Sunesis: Research Funding; Karyopharm: Research Funding; ImmunoGen: Consultancy; BMS: Research Funding; Novartis: Consultancy; Incyte: Consultancy; Sunesis: Consultancy; Alexion: Consultancy; Otsuka: Consultancy; Incyte: Research Funding. Short:Takeda Oncology: Consultancy. Cortes:Daiichi Sankyo: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Arog: Research Funding; Novartis: Consultancy, Research Funding. Kim:Amgen: Employment, Equity Ownership. Kelsh:Amgen: Employment, Equity Ownership. Katz:Amgen: Other: Former employee and stockholder; Kite, a GILEAD Company: Consultancy. Yang:Amgen Inc.: Employment, Equity Ownership. Mehta:Amgen: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.